Tumor Microenvironment Signaling and Therapeutics in Cancer Progression
- louise
- 799 Views
- Cancer Models, Cancer Therapy, Microfluidics, Therapeutic resistance, Tumor Microenvironment
Introduction
The review article discusses the critical role of the tumor microenvironment (TME) in cancer progression and therapeutic resistance. It highlights how the dynamic interplay between tumor cells and their surrounding microenvironment influences tumor growth, metastasis, and response to treatments.
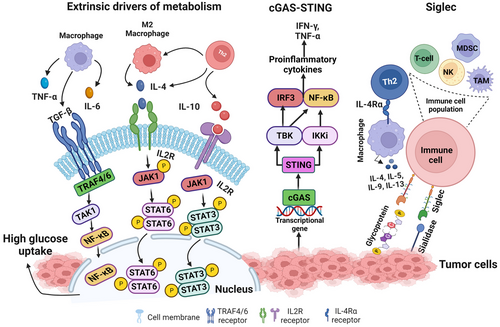
Tumor Microenvironment Components
The TME comprises various cellular and non-cellular components, including immune cells, stromal cells, extracellular matrix (ECM), and blood vessels. These elements interact with tumor cells, facilitating cancer progression through complex signaling networks.
Cancer-Associated Fibroblasts (CAFs):
CAFs are prominent in the TME and contribute to tumor growth and metastasis by remodeling the ECM, promoting angiogenesis, and modulating immune responses.
Immune Cells:
The TME contains diverse immune cells, including macrophages, T cells, and dendritic cells, which can either suppress or promote tumor progression depending on their state and the signals they receive from the tumor and stromal cells.
Extracellular Matrix (ECM):
The ECM provides structural support and biochemical signals that influence cell behavior. Alterations in the ECM composition and organization can enhance tumor cell invasion and resistance to therapies.
Tumor Endothelial Cells (TECs):
TECs form the blood vessels within tumors and differ from normal endothelial cells in their morphology and function. They support tumor growth by facilitating nutrient and oxygen supply and contributing to the immunosuppressive environment.
Models of the Tumor Microenvironment
The article discusses several models used to study the TME and its interactions with cancer cells:
2D Cell Culture Models:
These are traditional models where cells are grown in a monolayer. While useful, they lack the complexity of the TME found in vivo.
3D Culture Models:
These models involve growing cells in a three-dimensional structure, providing a more accurate representation of the TME. Examples include spheroid cultures and organoids, which mimic the architecture and cell-cell interactions found in tumors.
Microfluidic Models:
Also known as “organ-on-a-chip” models, these systems use microfluidics technology to recreate the dynamic environment of tissues, including the flow of fluids and the mechanical forces present in the TME. They allow for precise control over the microenvironment and can be used to study the interactions between different cell types within the TME.
Animal Models:
Mouse models, including genetically engineered mice and patient-derived xenografts (PDX), provide in vivo systems to study the TME. These models are crucial for understanding the complex interactions within the TME and for testing the efficacy of potential therapeutic strategies.
3D Bioprinting:
This technology involves layer-by-layer deposition of bioinks to create 3D structures that replicate the TME. It allows for the incorporation of multiple cell types and ECM components, providing a highly customizable platform for studying tumor biology and drug responses.
Signaling Pathways in TME
The review elaborates on key signaling pathways involved in TME interactions, including:
Hypoxia-Inducible Factor (HIF) Pathway:
Hypoxia within tumors stabilizes HIF, leading to the expression of genes that promote angiogenesis, metabolic adaptation, and survival under low oxygen conditions.
Transforming Growth Factor-beta (TGF-β) Pathway:
TGF-β signaling has dual roles, acting as a tumor suppressor in early stages and a promoter in advanced cancers by enhancing epithelial-mesenchymal transition (EMT) and immune evasion.
Immune Checkpoints:
Immune checkpoint molecules like PD-1/PD-L1 and CTLA-4 are upregulated in the TME, leading to immune suppression. Therapies targeting these checkpoints have shown promise in reinvigorating anti-tumor immune responses.
Therapeutic Strategies Targeting TME
The article reviews several therapeutic approaches aimed at modulating the TME to improve cancer treatment outcomes:
Anti-Angiogenic Therapies:
Drugs targeting VEGF and its receptors aim to disrupt the tumor blood supply. Although effective initially, tumors often develop resistance through alternative pro-angiogenic pathways.
CAF Targeting:
Strategies to inhibit CAF functions or deplete CAF populations are being explored to reduce tumor-promoting activities within the TME.
Immune Modulation:
Immunotherapies, including checkpoint inhibitors and CAR-T cell therapies, are designed to overcome the immunosuppressive TME and enhance anti-tumor immunity.
Matrix-Targeting Agents:
Approaches to degrade or normalize the ECM can improve drug delivery and reduce metastatic potential.
Clinical Implications and Future Directions
The review emphasizes the need for a deeper understanding of TME biology to identify novel therapeutic targets and develop more effective treatments. Combining TME-targeted therapies with conventional treatments may enhance therapeutic efficacy and overcome resistance.
Conclusion
The TME plays a pivotal role in cancer progression and therapeutic response. Comprehensive strategies that target multiple components of the TME hold promise for improving cancer treatment outcomes.
By integrating these insights, the article underscores the importance of continued research into the TME to advance cancer therapy and patient care.
Reference
For a detailed exploration of the review, refer to the full text Tumor microenvironment signaling and therapeutics in cancer progression, A. Goenka et. al, Cancer Communications.

 Job
Job Collaborations
Collaborations Customer
Customer Other
Other