Published on 03 May 2021
Single cell encapsulation background
To obtain meaningful biological data from single-cell experiments, the analysis of a large number of individual cells is required. This can be achieved using pressure-driven flow-controlled droplet-based microfluidics.
The microfluidic platform described in this application note is used to generate picoliter-sized microdroplets to encapsulate HeLa cells at the single cell level for further analysis. This process is called single cell encapsulation.
The cell suspension used has to have a concentration determined by a statistical distribution called the Poisson law, to obtain stable cell encapsulation during the experiment. This protocol can be adapted to encapsulate different types of cells and generate droplets of various sizes.
Applications
- Oncology (insight into mutations carried by small subpopulations of cells)
- Immunology (distinguishing different groups of immune cells or characterizing rare immune cell populations)
- Neurology (acquire a detailed single cell map to understand and identify different types of neurons and their connecting molecules in the brain)
- Developmental biology (mapping the entire developmental process of an embryo on a cell-by-cell basis)
- Single cell omics (uncover the cellular heterogeneity of a tissue sample)
- And many more possible applications
Single cell encapsulation setup
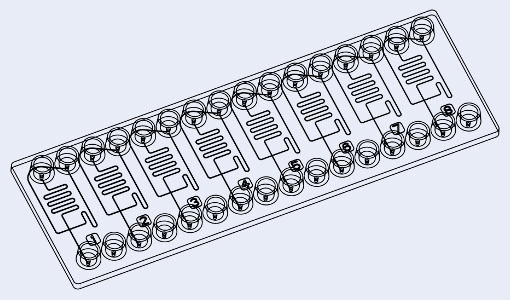
Fluidic 947 or fluidic 440 chip from microfluidic ChipShop Gmbh
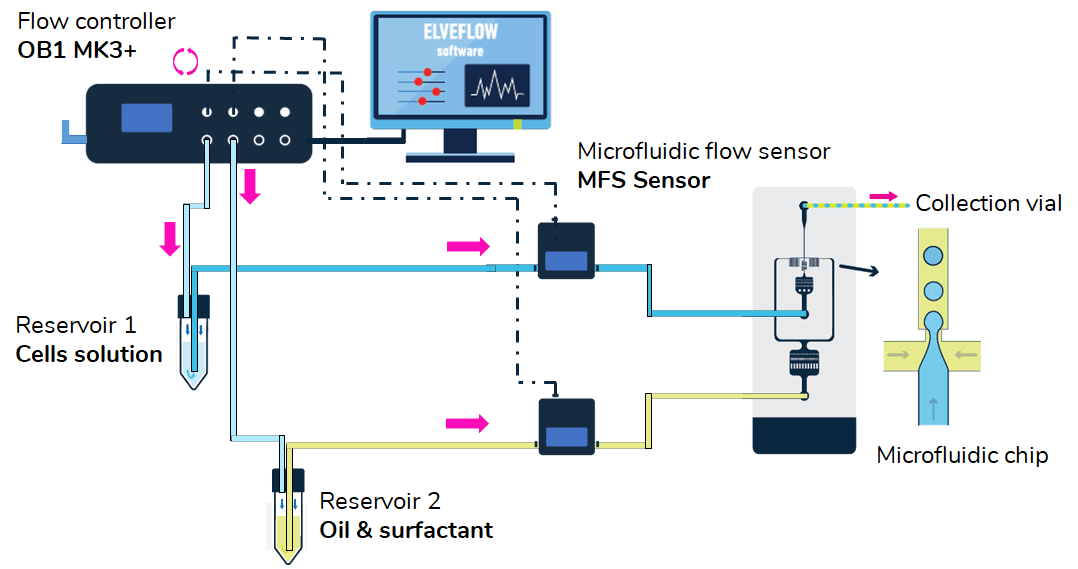
Hardware
- OB1 flow controller with at least two 0–2000 mbar channels
- 1 x Flow sensor MFS 2D 0.4–7 µL/min for the oil solution
- 1 x Flow sensor MFS MFS 3D 4,2–80 µL/min for the cell suspension
- 1 x Kit Luer Lock starter pack
- 2 x 15 mL falcon reservoirs
- Microfluidic chip with hydrophobic channels
- Microscope for observation
- High-speed camera for imaging (optional)
Chemicals
- HF5-7500 + 1 % FluoSurf surfactant (Emulseo, France)
- Aquapel (Autoserv, Germany) for the hydrophobic treatment (option)
[droplet_pack_rebound]
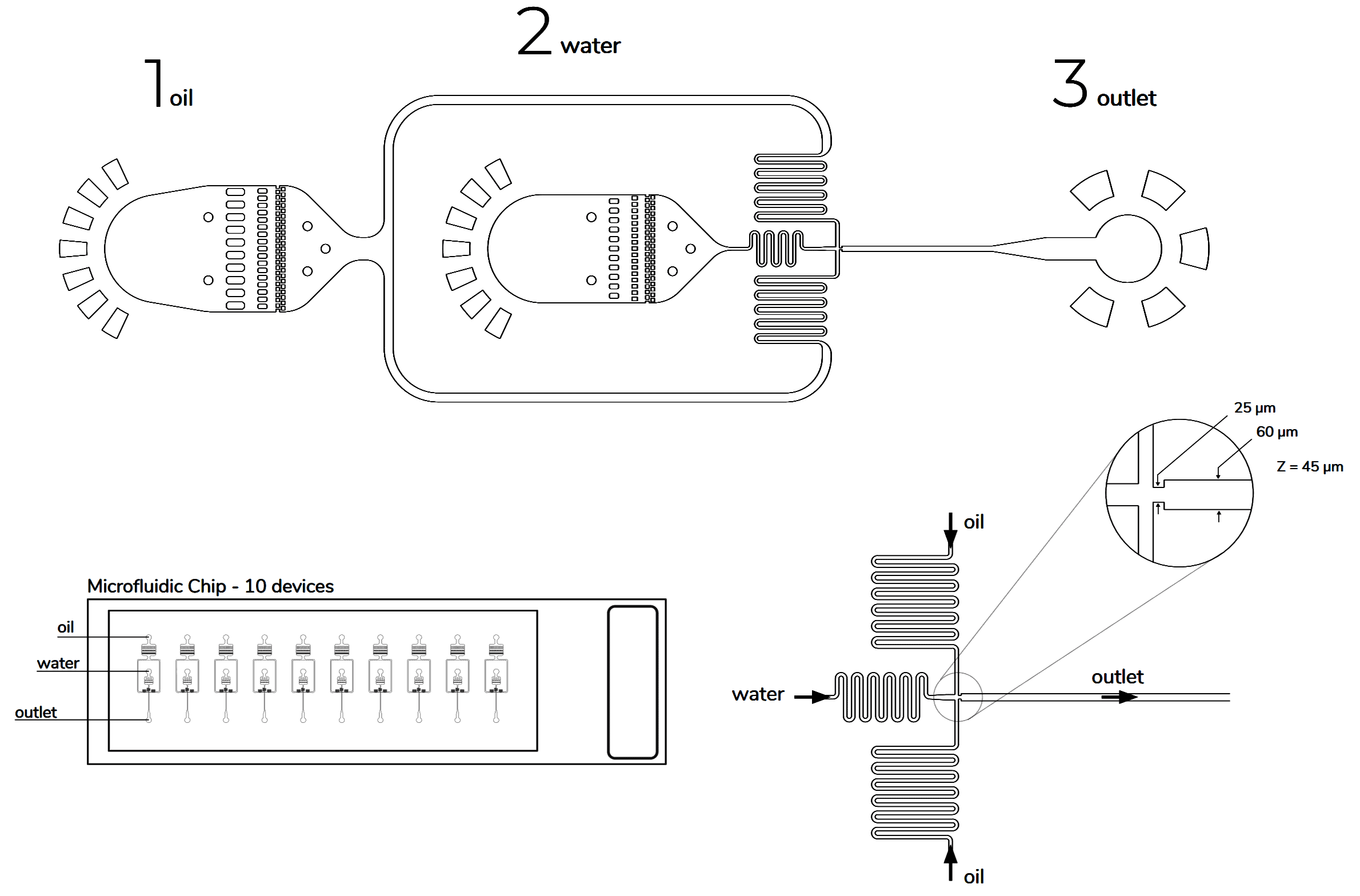
Design of the chip
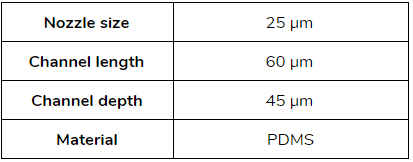
The microfluidic devices used have a flow focusing geometry and the chip is made of polydimethylsiloxane (PDMS). The microchannels are treated with Aquapel, resulting in hydrophobic channel surfaces.
Commercialized chips to ensure the same goal are available depending on the wanted sroplet size:
- Droplet size between [10 – 30] µm: microfluidic ChipShop (Fluidic 947 – Droplet generator chips)
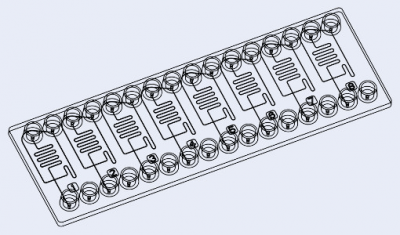
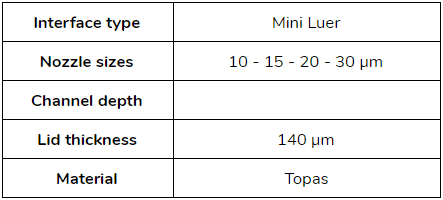
- Droplet size between [50 – 80] µm: microfluidic ChipShop (Fluidic 440 – Droplet generator chips)

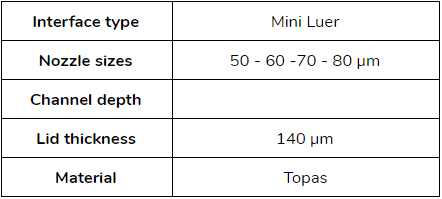
- Droplet size between [20 – 50] µm: Droplet genomics
For more information, contact us!
Quick Start Guide for single cell encapsulation
Instruments connections
- Connect your OB1 pressure controller to an external pressure supply using pneumatic tubing, and to a computer using a USB cable. For detailed instructions on OB1 pressure controller setup, please read the “OB1 User Guide”.
- Connect the flow sensors to the OB1.
- Turn on the OB1 by pressing the power switch.
- Launch the Elveflow software. The Elveflow Smart Interface’s main features and options are covered in the “ESI User Guide”. Please refer to the guide for a detailed description.
- Press Add instrument \ choose OB1 \ set as MK3+, set pressure channels if needed, give a name to the instrument and press OK to save changes. Your OB1 should now be on the list of recognized devices.
- OB1 calibration is required for the first use. Please refer to the “OB1 User Guide”.
- Add the flow sensors: press Add sensor \ select flow sensor \ analog or digital (choose the working range of flow rate for the sensor if you have an analog one), give a name to the sensor, select to which device and channel the sensor is connected and press OK to save the changes. Your flow sensor should be on the list of recognized devices. For details refer to “MFS user guide”.
- Open the OB1 Window.
Solution preparation
- Prepare the oil solution reservoir by adding a volume of HFE-7500 + 1 % FluoSurf surfactant and connect the supplied 1/16” OD tubing and the 4mm OD coil tubing to the tank. For more details, refer to the video “Connector for the OB1”.
- Repeat Step 1 for the aqueous solution prepared with a HeLa cell suspension for this experiment.
TIP: Prepare the HeLa cell suspension at a precise concentration calculated using the Poisson Law Distribution based on a specific droplet volume [1]. It is recommended to use a lower concentration to minimize the number of droplets containing two or more cells. A good measure of λ is between 0.05 and 0.1 (for λ = 0.05, although only 5% of all droplets will contain cells at all, 98% of these droplets will only contain a single cell).
- Option: to collect and reinject the emulsion of droplets, prepare a collecting vial filled with HFE-7500 + 1% of FluoSurf surfactant to keep the droplets in a stable environment after collection.
- If necessary, treat the microfluidic chip with an hydrophobic treatment, like aquapel (Autoserv, Germany).
TIP: Aquapel is a solution that crystallises when in contact with air. The best treatment is to flush the chip with: (i) argon, (ii) aquapel (iii) argon and (iv) HFE-7500 oil.
Single cell encapsulation
- For flow measurement, connect the flow sensors between the microfluidic reservoirs and the chip. For more details, refer to the “MFS User Guide”.
- Set a low pressure (50 mbar for example) of the oil solution until the solution starts dripping out of the tubing and then connect the tubing to the corresponding inlet. Fill the microfluidic chip with oil.
- Repeat Step 2 for the cell suspension.
TIP: To avoid cell sedimentation during experiment, a small agitation or vortex can be applied to the falcon tube. Different options are available depending on the type of cells and time of experiment.
- Apply a pressure of 300 mbar or a flow rate of 45 µL.min-1 for the oil solution and a pressure of 220 mbar or flow rate of 10 µL.min-1 for the cell suspension to obtain approximately 50 µm droplets.
TIP: The values given in step 4 can be increased to raise the generation frequency by keeping the ratio of flow rates.
- Collect the emulsion at the outlet via the collection vial for further experiments.
TIP: Make sure that the outlet is submerged in the oil and surfactant solution to ensure a stable environment for the droplets storage.
Single cell analysis
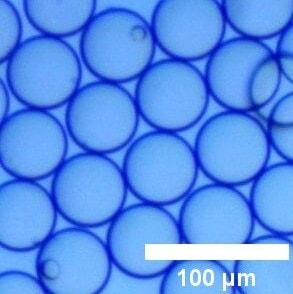
For this application note, a solution of cells (13.75 x 105 cells per mL) were encapsulated following the Poisson law distribution (𝜆 = 0.096) in an average droplet diameter of 51.2 ± 1.2 µm (CV = 2.4 %).
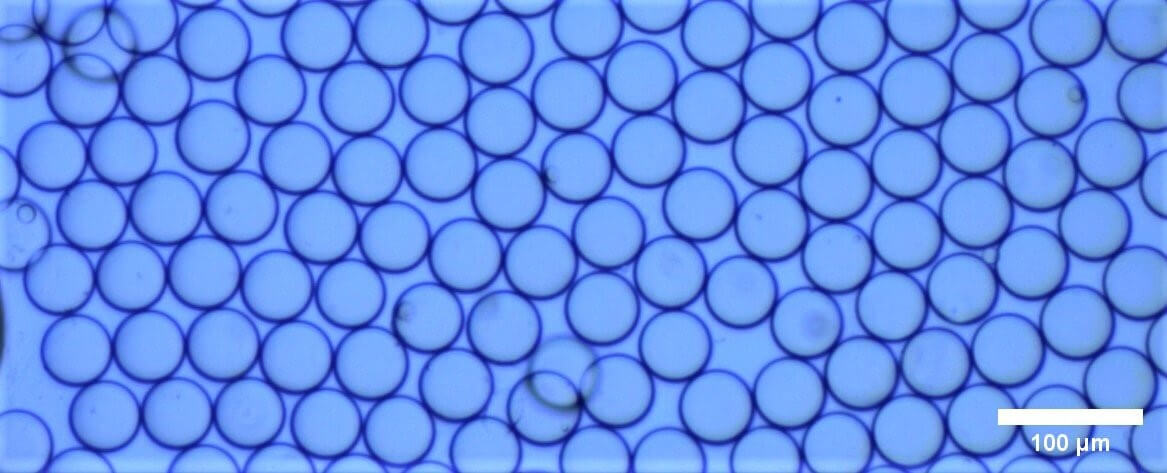
Figure 1: Generated droplets with an average diameter of 51.2 ± 1.2 µm. Four droplets each containing a single cell can be seen.
| Droplet diameter | 0 cell per droplet | 1 cell per droplet | ≥2 cells per droplet | |
| Theory | 50 µm | 91.4 % | 8.2 % | 0.4 % |
| Experimentally | 51.2 ± 1.2 µm | 90.9 % | 8.7 % | 0.4 % |
References
- Collins. D et al (2015), “The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation”. Lab on a Chip, 15, 3439-3459. https://pubs.rsc.org/en/content/articlelanding/2015/lc/c5lc00614g
Acknowledgements
Application note written by Robert BABER – Acknowledgement: This work was done thanks to the funding of European Union’s Horizon 2020 research and innovation programme ENHPATHY (H2020-MSCA-ITN, Grant agreement number: 860002)


Microfluidics knowledge
Do you want tips on how to best set up your microfluidic experiment? Do you need inspiration or a different angle to take on your specific problem? Well, we probably have an application note just for you, feel free to check them out!



 Job
Job Collaborations
Collaborations Customer
Customer Other
Other